New findings shed light on key stage of Plasmodium/malaria lifecycle
Published: 31 October 2017
New research on complex lifestyle identifies an important new target for future malaria treatments.
Malaria is one of the most devastating infections globally. Nearly half a million people die from this parasitic infection every year, while millions suffer ill health as a result. Most of the fatalities occur in children under the age of five.
Malaria is caused by the Plasmodium parasite, which has a highly complex lifecycle involving stages in both the Anopheles mosquito and the human host (see illustration). Different approaches to treatment will often target specific areas of the lifecycle including transmission to the mosquito. In order to be transmitted from an infected human to the mosquito vector, the parasites differentiate into male and female transmission forms known as gametocyctes. Gametocyte production rates and mosquito infection efficiency are highly variable but the underlying factors remain obscure.
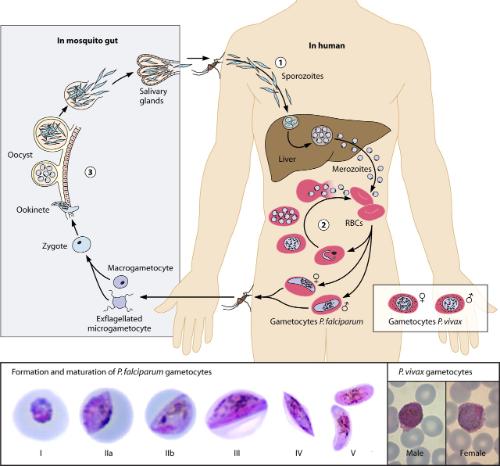
Plasmodium lifecycle (from: American Society of Microbiology)
New work by researchers at the Wellcome Centre for Molecular Parasitology, led by Professor Matt Marti, and colleagues at Harvard University have identified a key molecule that is found in human plasma, and which regulates gametocyte formation of the Plasmodium parasite. Variation in plasma levels of this molecule (known as LysoPC) during malaria infection therefore determines gametocyte numbers and ultimately transmission success. These results reveal that malaria parasites can sense and process host-derived physiological signals to regulate sexual differentiation and contribute new, critical knowledge of the biology of the Plasmodium parasite. Furthermore, they have helped to identify a major component of the sexual differentiation pathway, which may provide a new target for blocking malaria transmission.
This research was published today (November 9th) in the journal Cell.
First published: 31 October 2017
<< Centre News

